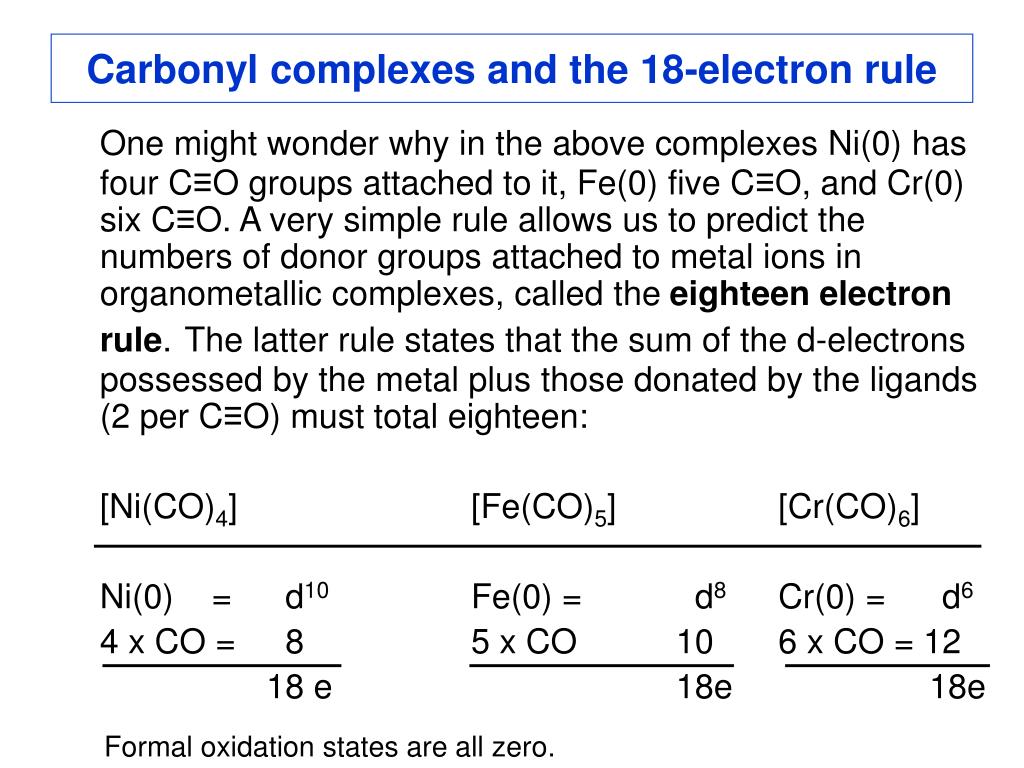
Fe2co9 18 Electron Rule

Diiron Nonacarbonyl Wikivisually
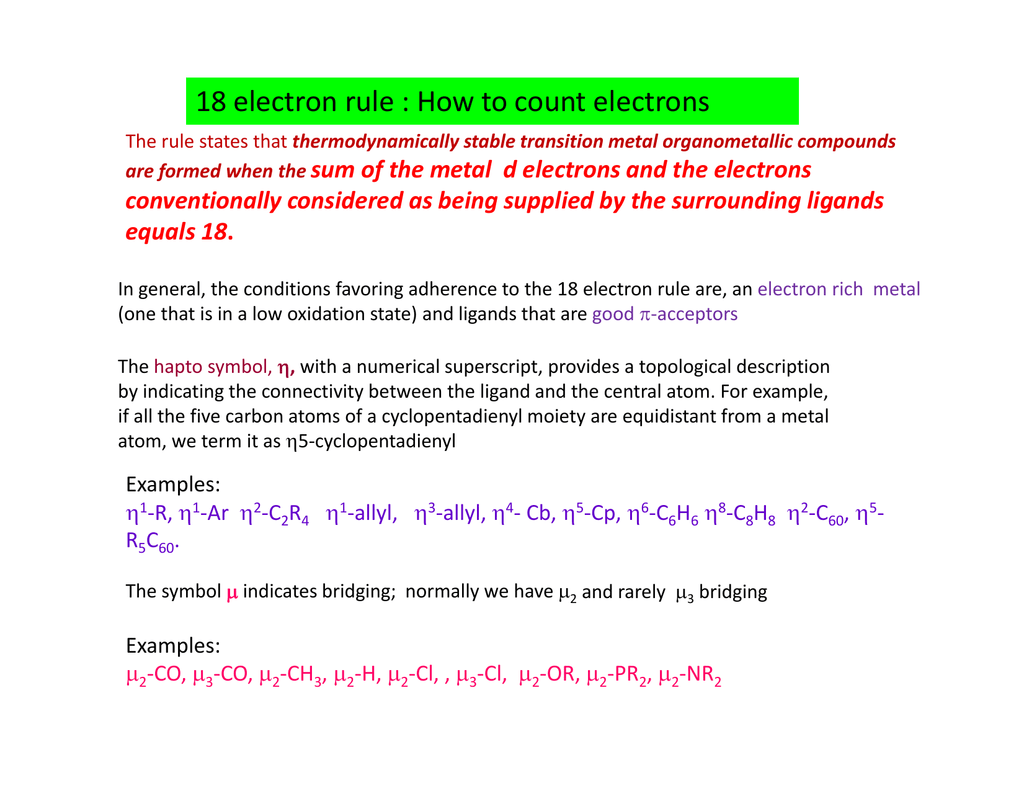
18 Electron Rule How To Count Electrons
24 3 The 18 Electron Rule Chemistry Libretexts

Metal Carbonyls

Metal Metal Bonding Bondslam
24 3 The 18 Electron Rule Chemistry Libretexts
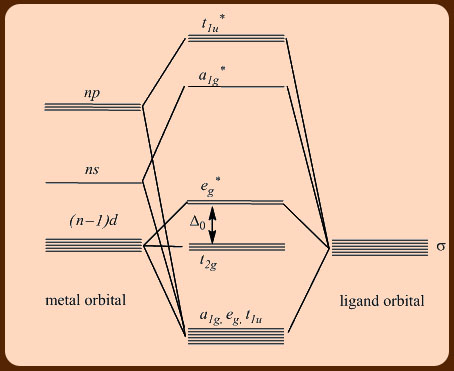
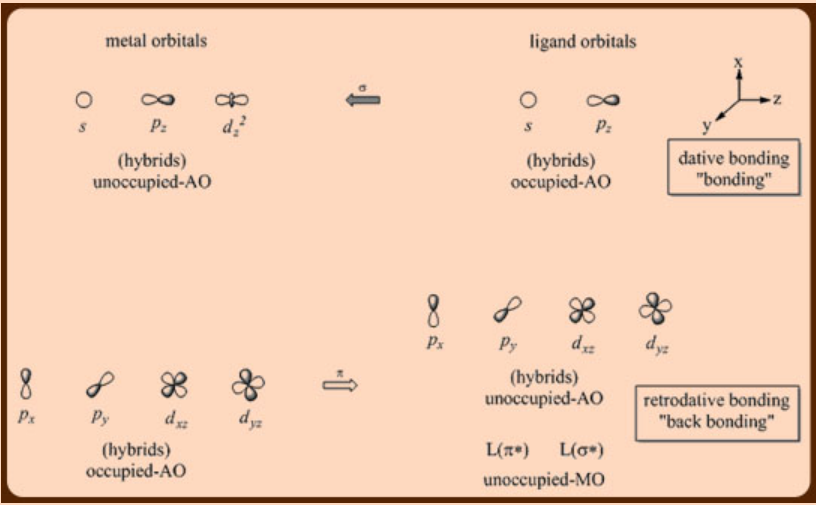
The donation of an electron pair to a metal cation is shown in figure \(\PageIndex{2}\) Figure \(\PageIndex{2}\) Binding of CO to a metal cation Remember, because the 18electron rule for transition metals makes them electrophilic, the electron pair does not need a positive charge to attract it (figure \(\PageIndex{3}\)).
Fe2co9 18 electron rule. Which obey the 18 electron rule?. The wellknown iron compound Fe2 with geometries suggestive of fourelectron donor carbo (CO)9 = Fe2(CO)6(lCO)3 is predicted by the 18elec nyl groups or metals with less than the favored 18elec tron rule to have an Fe–Fe single bond and is found tron configuration experimentally 38 to have an iron–iron distance of The computed. Electron Counting in Organometallic Chemistry 1 The 18Electron Rule;.
A Fe‐Fe bond (length 27(2) Å) is proposed for Fe 2 (μ‐TePh) 2 (NO) 4 on the basis of the 18‐electron rule The iron atom adopts a distorted tetrahedral geometry with acute bridge Fe‐Te‐Fe angles 6791(3)°, and bridging Fe‐Te bond of length 253(1) Å. Nomenclature (m, h), coordination number and electron counting Lecture 2 Why complexes form 18electron rule Recap of molecular orbital theory sdonor ligands (hydride complexes) Construction and interpretation of octahedral ML 6 molecular orbital energy diagram Lecture 3 pacceptor ligands, synergic bonding, CO, CN, N 2, Lecture 4. Transition Metal Carbonyl Cluster Chemistry describes models and rules that correlate cluster structure with electron count, which are then applied in worked examples Subsequent chapters explain how bonding relates to molecular structure, demonstrate the use of spectroscopic techniques such as NMR, IR and MS in.
18इलेक्ट्रॉन नियम , पेन्टा कार्बोनिल आयन , 18electron rule in hindi हेक्सा कार्बोनिल क्रोमियम (CO)10 , Fe2(CO)9 नोट धातु धातु (MM) बंध के लिए प्रत्येक धातु का 1. Thus the loss of CO ligands from these binuclear complexes leads to an increase in the hapticity of the acenaphthylene ligand, as expected by consideration of the 18electron rule The low CO dissociation energy of B5 kcal molÀ1 in going from (C12H8)Fe2(CO)6 to (C12H8)Fe2(CO)5 can relate to the energy released in forming an ironiron bond. Note that when writing the electron configuration for an atom like Fe, the 3d is usually written before the 4s For the Fe2 ion we remove two electrons from 4s2 leaving us with 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 For the Fe3 ion we remove a total of three electrons (two from the 4s2 and one form the 3d6) leaving us with 1s 2 2s 2 2p 6 3s 2 3p.
This strategy is exploited to review the controversial existence of direct Fe−Fe bonding in the triply bridged Fe2(CO)9 system Although the bond is predicted by electron counting rules, the interaction between the two pseudooctahedral metal centers can be repulsive because of their fully occupied t2g sets. The number of CO ligands considered to the metal is generally in accordance with the 18electron rule and this rule is followed by 99% of the metal carbonyls These contain metal atoms of only one elementFor example Fe2(CO)9,Mn2(CO)10,Co2(CO)8,Fe3(CO)12,Co4(CO)12,Rh4(CO)12,Ir4(CO)12 etc. The 18 electron rule allows one to predict the reactivity of a certain compound The associative mechanism means that there is an addition of a ligand while a dissociative mechanism means that there is a loss of a ligand When the electron count is less than 18, a molecule will most likely undergo an associative reaction.
Total electron count 18 Co3 comply with the 18VE rule and is thus a coordinatively unsaturated 16 electron species ix 9 Explain the below trend in CO bond vibrational frequency v(CO) using the Dewar‐Chatt. All of the Co−Co bond distances in the Co 4 (CO) 12 neutral have similar values (around 250 Å) and are consistent with the single bonds required by the 18 electron rule. Nomenclature (m, h), coordination number and electron counting Lecture 2 Why complexes form 18electron rule Recap of molecular orbital theory sdonor ligands (hydride complexes) Construction and interpretation of octahedral ML 6 molecular orbital energy diagram Lecture 3 pacceptor ligands, synergic bonding, CO, CN, N 2, Lecture 4.
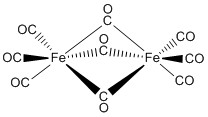
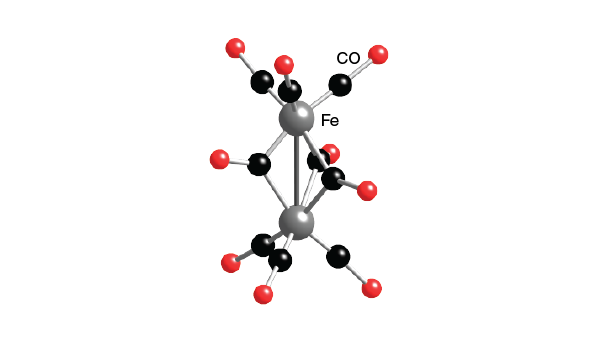
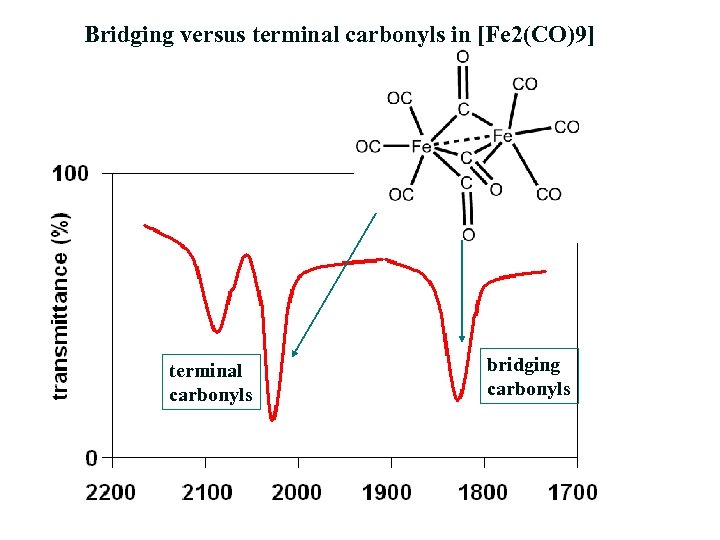
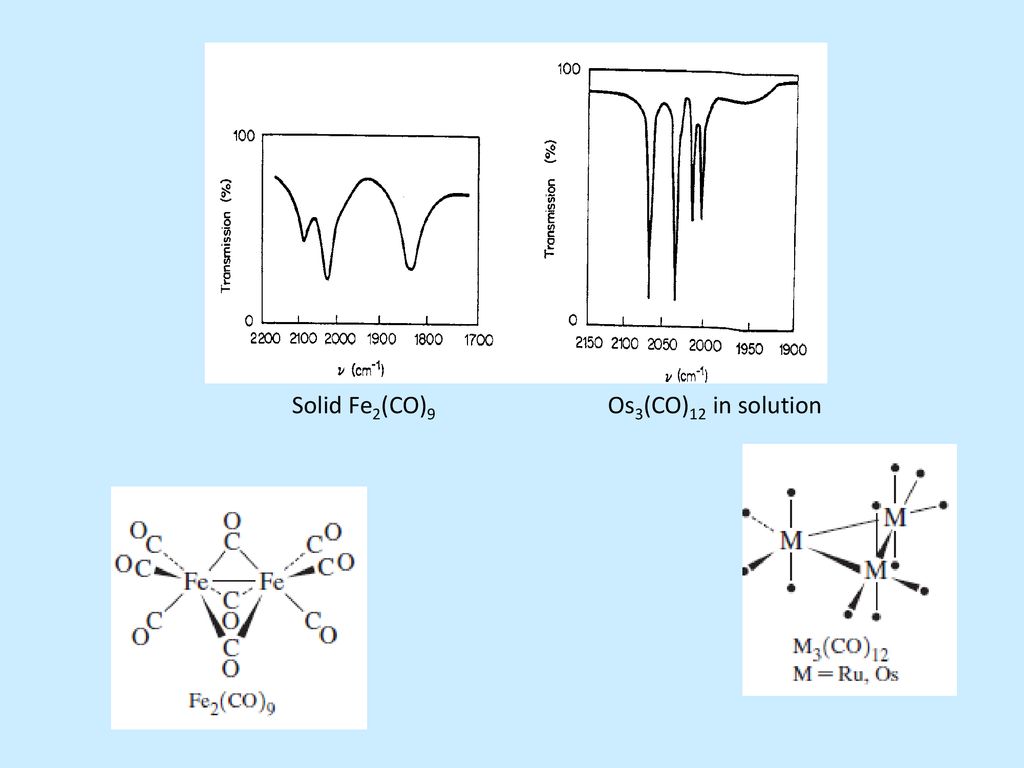
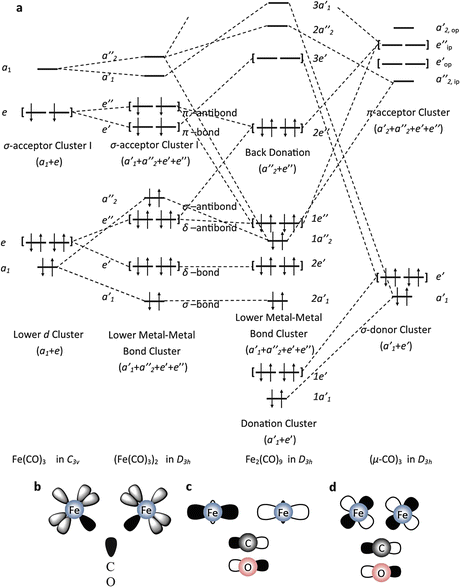
Thus C68 I obeys the 18electron rule and is similar to Fe3 (CO)9 As2 and Fe3 (CO)10 (NSiMe3) The ironron and ironulfur bond lengths in I are very close to those in II and III Howe~er because of the presence of two more electrons, II and III contain open triangles of metal atoms with only two metaletal bonds. E) Cr(CO) 6 at 00 cm1 vs (C 6 H 5 CH 3)Cr(CO) 3 at 1963 and 1869 cm1 Problem MI26 Sometimes, carbonyls can bridge between two metals For example, the iron cluster Fe2(CO)9 contains six "terminal" carbonyls, bound to only one iron each, and three "bridging" carbonyls, each of which is bound to both iron atoms. Synthesis and structure Following the original method, photolysis of an acetic acid solution of Fe(CO) 5 produces Fe 2 (CO) 9 in good yield 2 Fe(CO) 5 → Fe 2 (CO) 9 CO Fe 2 (CO) 9 consists of a pair of Fe(CO) 3 centers linked by three bridging CO ligands Although older textbooks show an FeFe bond consistent with the 18 electron rule (8 valence electrons from Fe, two each from the.
A Fe‐Fe bond (length 27(2) Å) is proposed for Fe 2 (μ‐TePh) 2 (NO) 4 on the basis of the 18‐electron rule The iron atom adopts a distorted tetrahedral geometry with acute bridge Fe‐Te‐Fe angles 6791(3)°, and bridging Fe‐Te bond of length 253(1) Å. FEFE bridging give 1 electron CO give 2 electron thus 9 CO X 2 = 18 electrons since FE 0 oxidation state thus = 4S2 3D6 = 8 electrons , got two Fe here so 8x2= 16 e overal total inlcuding the bridging between FeFe = 1e 16e 18e = 35 electrons. 18 electron Rule or Effective Atomic Number (EAN) Rule Meet some new ligandsPPh3PR3 MeCN, thf, MeOH (donor solvents) diphos or dppe = Ph2P(CH2)2PPh2X, Cl, Br, OH, SRMo(CO)5(PPh3) Organometallic Chemistry Concepts1 18 electron Rule or Effective Atomic Number (EAN) Rule Can you think of other possible ligands for organometallics.
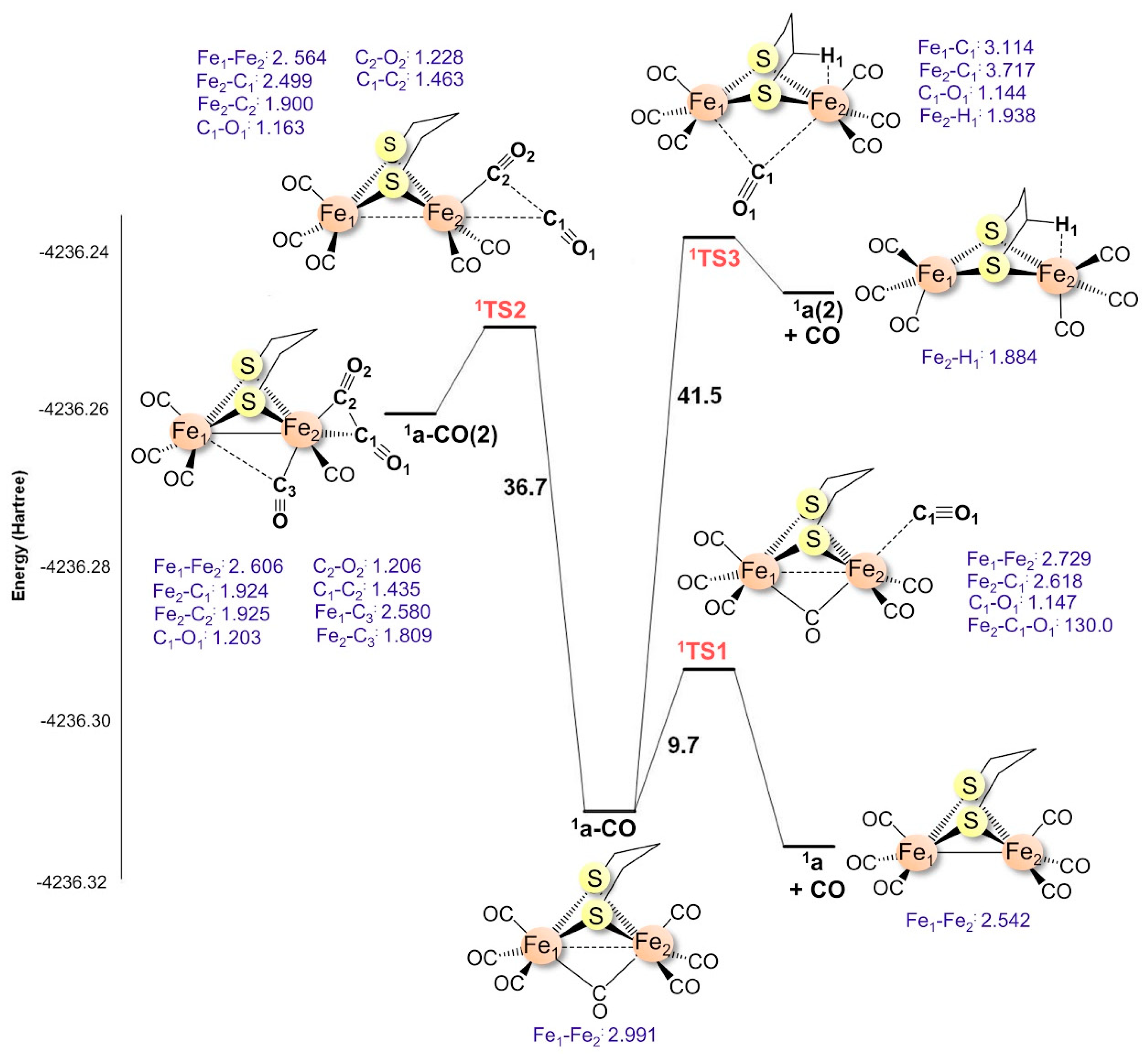
Definition & rationalisation The constitution and structure of main group element complexes can be predicted and rationalised by a combination of the "Octet Rule" and VSEPR eg SnMe4,BH3NH3 In transition metal (TM) complexes, the "18 Electron Rule" generally applies. The 18electron rule and its exceptions (1) Octahedral complexes à can be split into 3 categories category number of electrons description A 18 18electron rule obeyed B 1218 18electrons not exceeded C 1222 18electron rule disobeyed A Those that obey the 18 electron rule Complexes with strong πacceptor ligands (eg V(CO)6, Cr(CO)6. In this research, we consider homoleptic iron carbonyls that satisfy the 18electron rule but may have formal double Fe2(CO)8 (seven distinct structures), triple Fe2(CO)7 (three distinct.
FeCCO)9 Ei Fe (CO)3 (X) The reaction of 3,4dihalocyclobutenes with Fe2(CO)9 now appears to be a general method for the preparation of cyclobutadiene iron tricarbonyl complexes Thus, as indicated in the following reactions, the Fe(CO)3 complexes of 1,2diphenylcyclobutadiene (XI), 1,2,3,4tetramethylcyclo. Diiron nonacarbonyl, F e X 2 (C O) X 9, is often depicted with an Fe–Fe bond as shown at the lefthand side The Fe—Fe bond is usually invoked in order to (1) explain the observed diamagnetism and (2) satisfy the 18electron rule. Thus the loss of CO ligands from these binuclear complexes leads to an increase in the hapticity of the acenaphthylene ligand, as expected by consideration of the 18electron rule The low CO dissociation energy of B5 kcal molÀ1 in going from (C12H8)Fe2(CO)6 to (C12H8)Fe2(CO)5 can relate to the energy released in forming an ironiron bond.
Total electron count 18 Co3 comply with the 18VE rule and is thus a coordinatively unsaturated 16 electron species ix 9 Explain the below trend in CO bond vibrational frequency v(CO) using the Dewar‐Chatt. All of the Co−Co bond distances in the Co 4 (CO) 12 neutral have similar values (around 250 Å) and are consistent with the single bonds required by the 18 electron rule. The 18electron rule and its exceptions (1) Octahedral complexes à can be split into 3 categories category number of electrons description A 18 18electron rule obeyed B 1218 18electrons not exceeded C 1222 18electron rule disobeyed A Those that obey the 18 electron rule Complexes with strong πacceptor ligands (eg V(CO)6, Cr(CO)6.
The number of CO ligands considered to the metal is generally in accordance with the 18electron rule and this rule is followed by 99% of the metal carbonyls These contain metal atoms of only one elementFor example Fe2(CO)9,Mn2(CO)10,Co2(CO)8,Fe3(CO)12,Co4(CO)12,Rh4(CO)12,Ir4(CO)12 etc. Although older textbooks show an FeFe bond consistent with the 18 electron rule (8 valence electrons from Fe, two each from the terminal carbonyls, one each from the bridging carbonyls and one from the other Fe atom in the metalmetal bond), theoretical analyses have consistently indicated the absence of a direct FeFe bond this latter model proposes an FeCFe threecentertwoelectron " banana bond " for one of the bridging carbonyls. We describe herein the structure of a bi nuclear complex Fe2 (CO)6 (C6F5 )2P (C6 F5 )2PC4 (Ph)2 1 (1) in which a hetero cyclic 3,4dihapto1phosphoniacyclopentadiene ligand formed in the reaction of the phosphinoacetylene (C6F5)2PC~CPh with Fe2 (CO)9 is coordinated as a 3 electron donor to a duron hexacarbonyl moiety.
This strategy is exploited to review the controversial existence of direct Fe−Fe bonding in the triply bridged Fe2(CO)9 system Although the bond is predicted by electron counting rules, the interaction between the two pseudooctahedral metal centers can be repulsive because of their fully occupied t2g sets. The homoleptic binuclear compound Fe2(CO)9 is well characterized experimentally, although there has been some discussion as to the nature of the iron−iron bond, which is at most a single bond In this research, we consider homoleptic iron carbonyls that satisfy the 18electron rule but may have formal double Fe2(CO)8 (seven distinct structures), triple Fe2(CO)7 (three distinct structures. Electron Counting in Organometallic Chemistry 1 The 18Electron Rule;.
Definition & rationalisation The constitution and structure of main group element complexes can be predicted and rationalised by a combination of the "Octet Rule" and VSEPR eg SnMe4,BH3NH3 In transition metal (TM) complexes, the "18 Electron Rule" generally applies. The 18 electron rule holds for each Fe atom as Fe(0) = d8 3 COs = 6 3 bridge COs = 3 FeFe FeFe bond = 1 bond Fe2(CO)9 18 e Charged ligands and the eighteenelectron rule The formally charged ligands that are important in. This strategy is exploited to review the controversial existence of direct Fe−Fe bonding in the triply bridged Fe2(CO)9 system Although the bond is predicted by electron counting rules, the interaction between the two pseudooctahedral metal centers can be repulsive because of their fully occupied t2g sets.
Interactive 3D chemistry animations of reaction mechanisms and 3D models of chemical structures for students studying University courses and advanced school chemistry. YES 6 Compounds and the EAN Rule We can divide compounds into three groups 1 Electronic configurations are completely unrelated to the EAN rule The central metal may have >, 18 electrons, but may have less 3. Interactive 3D chemistry animations of reaction mechanisms and 3D models of chemical structures for students studying University courses and advanced school chemistry.
Today, in my course I talked about the 18 electronrule and gave the students a few problems to practice I asked the students to determine if the complex Fe2(CO)9 followed the 18 electron rule So, I go through the example and explain the the MM bond contributes one electron. Application of the 18electron rule The majority of metal complexes do not satisfy the 18electron ruleIt is, however, especially useful for organometallic complexes of the Cr, Mn, Fe, and Co triads, and applies to compounds such as ferrocene, iron pentacarbonyl, chromium carbonyl and nickel carbonylIn compounds such as these, the nine bonding molecular orbitals are all low in energy. Thus C68 I obeys the 18electron rule and is similar to Fe3 (CO)9 As2 and Fe3 (CO)10 (NSiMe3) The ironron and ironulfur bond lengths in I are very close to those in II and III Howe~er because of the presence of two more electrons, II and III contain open triangles of metal atoms with only two metaletal bonds.
The wellknown iron compound Fe2 with geometries suggestive of fourelectron donor carbo (CO)9 = Fe2(CO)6(lCO)3 is predicted by the 18elec nyl groups or metals with less than the favored 18elec tron rule to have an Fe–Fe single bond and is found tron configuration experimentally 38 to have an iron–iron distance of The computed. The homoleptic binuclear compound Fe2(CO)9 is well characterized experimentally, although there has been some discussion as to the nature of the iron−iron bond, which is at most a single bond In this research, we consider homoleptic iron carbonyls that satisfy the 18electron rule but may have formal double Fe2(CO)8 (seven distinct structures), triple Fe2(CO)7 (three distinct structures. In this research, we consider homoleptic iron carbonyls that satisfy the 18electron rule but may have formal double Fe2(CO)8 (seven distinct structures), triple Fe2(CO)7 (three distinct.
The 18 electron rule holds for each Fe atom as Fe(0) = d8 3 COs = 6 3 bridge COs = 3 FeFe FeFe bond = 1 bond Fe2(CO)9 18 e Charged ligands and the eighteenelectron rule The formally charged ligands that are important in. The donation of an electron pair to a metal cation is shown in figure \(\PageIndex{2}\) Figure \(\PageIndex{2}\) Binding of CO to a metal cation Remember, because the 18electron rule for transition metals makes them electrophilic, the electron pair does not need a positive charge to attract it (figure \(\PageIndex{3}\)).

Structure And Bonding In Binuclear Metal Carbonyls From The Analysis Of Domain Averaged Fermi Holes I Fe2 Co 9 And Co2 Co 8 Ponec 08 Journal Of Computational Chemistry Wiley Online Library
Ppt Lecture An Introduction To Organometallic Chemistry Powerpoint Presentation Id

Is There An Iron Iron Bond In Diiron Nonacarbonyl Chemistry Stack Exchange

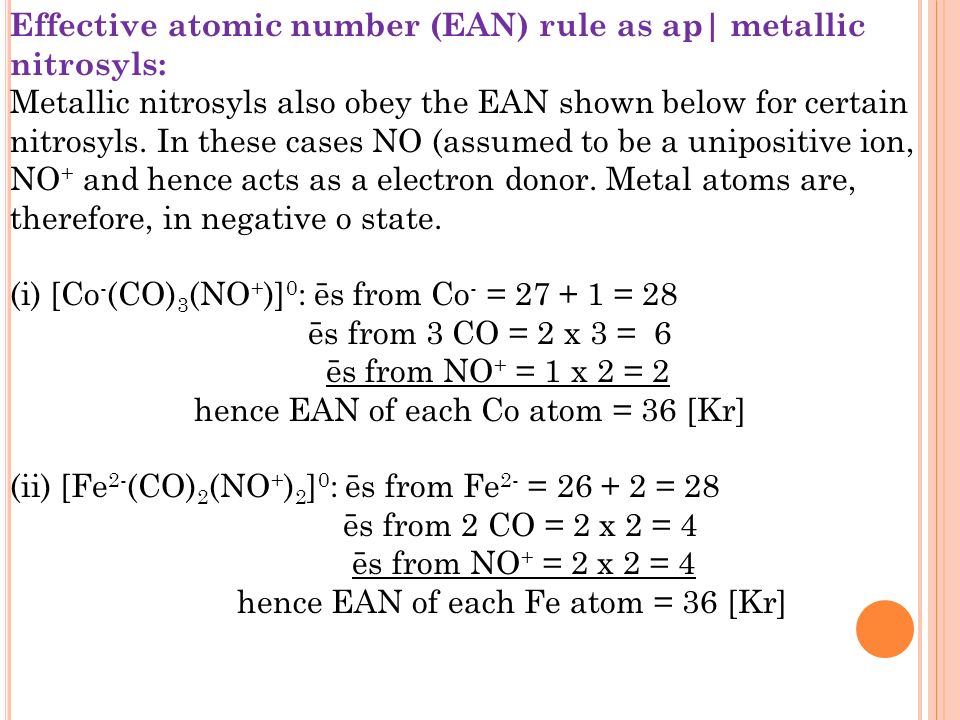
Metallic Carbonyls And Metallic Nitrocyls Ppt Video Online Download

Lecture An Introduction To Organometallic Chemistry Ppt Video Online Download

Cyclopentadienyliron Dicarbonyl Dimer Wikipedia
Chm 501 Lecture 19 Organometallic Chemistry
Htiovztslgootm

Metal Metal Bonding Bondslam

Is There An Iron Iron Bond In Diiron Nonacarbonyl Chemistry Stack Exchange
Unsaturation In Binuclear Heterometallic Carbonyls The Cyclopentadienyliron Manganese Carbonyl Cpfemn Co N System As A Hybrid Of The Cp2fe2 Co N And Mn2 Co N Systems New Journal Of Chemistry Rsc Publishing
Welcome To Chem Zipper Com Sidwick Theory And Ean Rule

The Experimental And Simulated Vibrational Spectra Of The Fe 2 Co 8 Download Scientific Diagram

Organometallic Flashcards Quizlet

Lecture An Introduction To Organometallic Chemistry Ppt Video Online Download

Pdf Molecular Structures Of M2 Co 9 And M3 Co 12 M Fe Ru Os New Theoretical Insights Carlo Mealli Academia Edu

High Resolution X Ray Absorption Spectroscopy Of Iron Carbonyl Complexes Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C5cpd
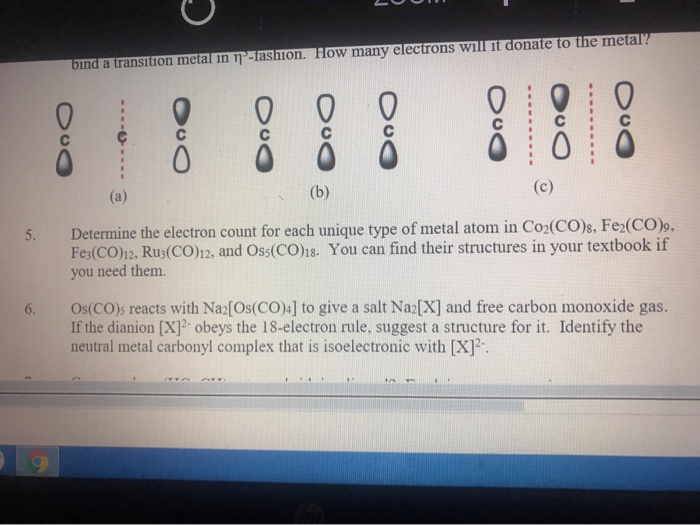
Solved Bind A Transition Metal In Tashion How Ma 5 Det Chegg Com
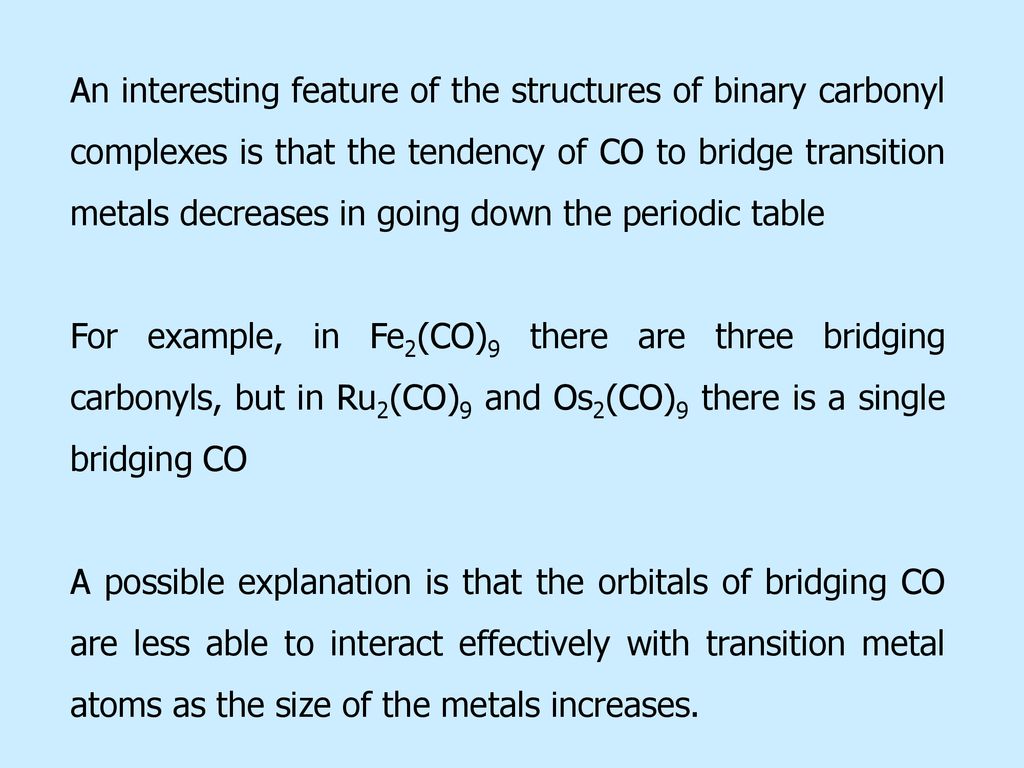
Metal Carbonyls Ppt Download

Is There An Iron Iron Bond In Diiron Nonacarbonyl Chemistry Stack Exchange

Effective Atomic Number Rule Online Chemistry Tutorial That Deals With Chemistry And Chemistry Concept

Metal Carbonyl Wikipedia

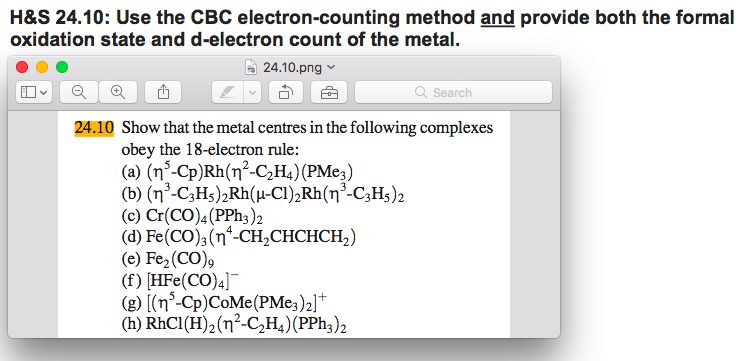
Solved Show That The Metal Centers In The Following Compl Chegg Com

18 Electron Rule How To Count Electrons
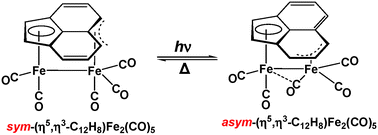
The Hapticity Of The Acenaphthylene Ligand In Its Mononuclear Binuclear And Trinuclear Iron Carbonyl Complexes New Journal Of Chemistry Rsc Publishing
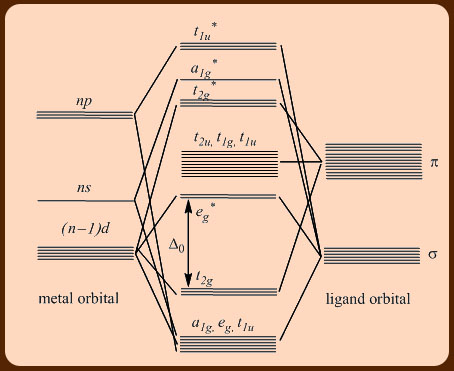
24 3 The 18 Electron Rule Chemistry Libretexts
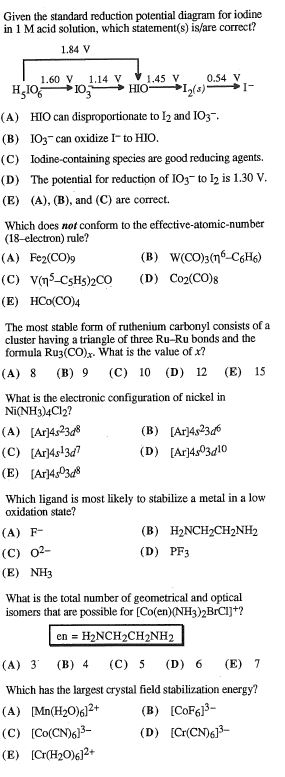
Solved I Need To Check My Answers To These Problems Plea Chegg Com

Triiron Dodecacarbonyl Wikipedia

Structures Interconversions And Spectroscopy Of Iron Carbonyl Clusters With An Interstitial Carbide Localized Metal Center Reduction By Overall Cluster Oxidation Abstract Europe Pmc

Organometallic Flashcards Quizlet
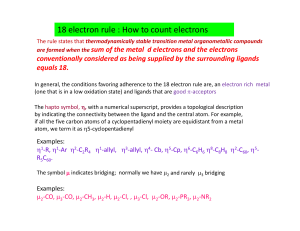
18 Electron Rule How To Count Electrons

Metal Carbonyls

Pdf The 18 Electron Rule And Electron Counting In Transition Metal Compounds Theory And Application Semantic Scholar
Phase Diagram And Transformations Of Iron Pentacarbonyl To Nm Layered Hematite And Carbon Oxygen Polymer Under Pressure Scientific Reports

The Experimental And Simulated Vibrational Spectra Of The Fe 2 Co 9 Download Scientific Diagram

Ktt211 18 Electron Rules Pdf Ionic Bonding Coordination Complex

High Resolution X Ray Absorption Spectroscopy Of Iron Carbonyl Complexes Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C5cpd
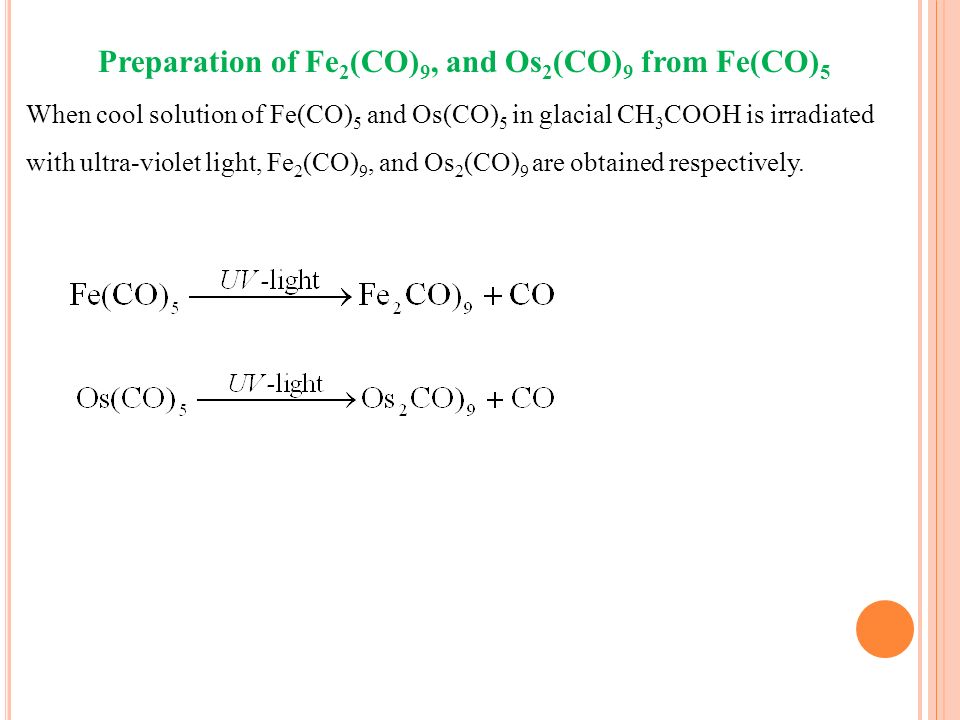
Metallic Carbonyls And Metallic Nitrocyls Ppt Video Online Download

Chapter 10

Is There An Iron Iron Bond In Diiron Nonacarbonyl Chemistry Stack Exchange

Structure And Bonding In Binuclear Metal Carbonyls From The Analysis Of Domain Averaged Fermi Holes I Fe2 Co 9 And Co2 Co 8 Ponec 08 Journal Of Computational Chemistry Wiley Online Library

Pentacarbonyliron An Overview Sciencedirect Topics

Optimized Structures Of The Low Lying Fe 2 Co 8 Isomers De Values Download Scientific Diagram

Metal Carbonyl Wikipedia

Structures Interconversions And Spectroscopy Of Iron Carbonyl Clusters With An Interstitial Carbide Localized Metal Center Reduction By Overall Cluster Oxidation Abstract Europe Pmc

Oneclass Photolysis Of Fe Co 5 Results In Loss Of Co And The Generation Of Several Metal Carbonyl Cl

Organometallic Chemistry Ppt Video Online Download

Carbonyl Complexes Coordination Complex Ligand

Cyclopentadienyliron Dicarbonyl Dimer Wikipedia
6 1 18 Valence Electron Rule Chemistry Libretexts
Co 9 And Os 2 Co 9 From Fe Co 5 Studyslide Com
Fe2 Co 9 Diiron Nonacarbonyl

Effective Atomic Number Of Fe In Fe2 Co 9 Is Brainly In
What Is The Ean Value Of Fe2 Co 9 Quora

슬라이드 1 Yonsei University Powerpoint Presentation Free Online Download Ppt Qkyqbg

Structure And Bonding In Binuclear Metal Carbonyls From The Analysis Of Domain Averaged Fermi Holes I Fe2 Co 9 And Co2 Co 8 Ponec 08 Journal Of Computational Chemistry Wiley Online Library
Organometallic Chemistry 1 Introduction Types And Rationale 2

Solved D Fe2 Co 9 3 Determine The Metal Metal Bond Ord Chegg Com

Structures Interconversions And Spectroscopy Of Iron Carbonyl Clusters With An Interstitial Carbide Localized Metal Center Reduction By Overall Cluster Oxidation Abstract Europe Pmc

Iron Pentacarbonyl Wikipedia

24 3 The 18 Electron Rule Chemistry Libretexts

24 3 The 18 Electron Rule Chemistry Libretexts

Organobismuth Compound An Overview Sciencedirect Topics
Solved H S 24 10 Use The Cbc Electron Counting Method An Chegg Com

Diiron Nonacarbonyl Wikipedia

Optimized Structures Of The Low Lying Fe 2 Co 9 Isomers De Values Download Scientific Diagram
Metal Carbonyls Ppt Download

Organometallic Chemistry Ppt Download
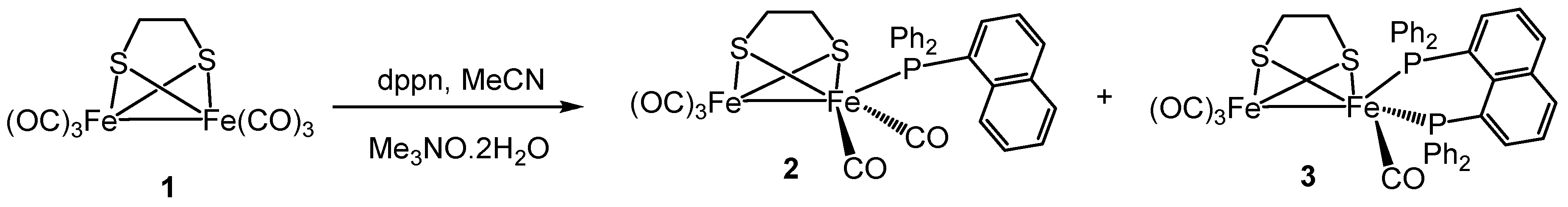
Inorganics Free Full Text Hydrogenase Biomimetics With Redox Active Ligands Synthesis Structure And Electrocatalytic Studies On Fe2 Co 4 K2 Dppn µ Edt Edt Ethanedithiolate Dppn 1 8 Bis Diphenylphosphino Naphthalene Html
Metallic Carbonyls And Metallic Nitrocyls Ppt Video Online Download

Lecture An Introduction To Organometallic Chemistry Ppt Video Online Download
The Rich Structural Chemistry Displayed By The Carbon Monoxide As A Ligand To Metal Complexes Springerlink
Effective Atomic Number Of Fe In Fe 2 Co 9 Is Socratic

Pdf High Resolution X Ray Absorption Spectroscopy Of Iron Carbonyl Complexes
6 4 Organometallic Chemistry Of D Block Metals Part 1 Chemistry Libretexts



